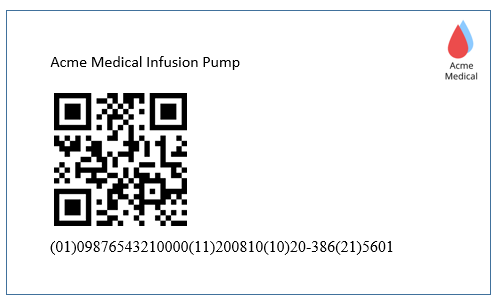
Scan the Unique Device Identifier (UDI) barcode - instantly identifying the manufacturer, device model and serial / batch.
Register your hospital, and report issues via interactive form, attach photos, videos, or audios, and communicate with the support team.
Track progress and get timely updates for a fast resolution.

UDILink enables easy, instant and accurate user reporting of medical device complaints and feedback to manufacturers as required by the FDA and similar authorities.
1. Scan UDI on medical device.
Don't have a medical device in front of you? Try with our demo UDI here.
2. Record and describe problem, and upload to manufacturer.
3. Quicker communications and updates

Obtain more and higher quality postmarket feedback, improving product safety, performance.

Quick efficient accurate complaint and feedback reporting platform for users - field engineers, hospital nurses, unit managers, technicians, service staff, consumers.

Scan the UDI code which is located on the problematic medical device or use the sample code as below